This test is provided to determine compliance with drug-release requirements where specified in individual monographs. Use the apparatus specified in the individual monograph. Replace the aliquots withdrawn for analysis with equal volumes of fresh
Dissolution Medium at 37
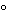
or, where it can be shown that replacement of the medium is not necessary, correct for the volume change in the calculation.
[NOTE—Medium replacement is not necessary for
Apparatus 4, which is a continuous-flow system.
] Keep the vessel covered for the duration of the test, and verify the temperature of the mixture under test at suitable times.